I-EB Virus Nucleic Acid
Igama lomkhiqizo
I-HWTS-OT061-EB Virus Nucleic Acid Detection Kit(Fluorescence PCR)
Isitifiketi
CE
I-Epidemiology
I-EBV (i-Epstein-barr virus), noma uhlobo lwe-herpesvirus yomuntu 4, i-herpesvirus yomuntu evamile.Eminyakeni yamuva nje, inani elikhulu lezifundo liye lafakazela ukuthi i-EBV ihlotshaniswa nokuvela nokuthuthukiswa komdlavuza we-nasopharyngeal, isifo sikaHodgkin, i-T/Natural killer celllymphoma, i-lymphoma ye-Burkitt, umdlavuza webele, umdlavuza wesisu kanye nezinye izimila ezimbi.Futhi kuhlotshaniswa kakhulu nokuphazamiseka kwe-post-transplantlymphoproliferative, isimila esibushelelezi se-post-transplant kanye ne-immunedeficiency syndrome ehlobene ne-AIDS (AIDS), i-multiple sclerosis, i-primary central central nervous system lymphoma noma i-leiomyosarcoma.
Isiteshi
| FAM | I-EBV |
| I-VIC (HEX) | Ukulawula kwangaphakathi |
Imingcele Yezobuchwepheshe
| Isitoreji | ≤-18℃ ebumnyameni |
| Impilo yeshelufu | Izinyanga ezingu-12 |
| Uhlobo lwesifanekiso | Igazi eliphelele, i-Plasma, iSerum |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 500Amakhophi/mL |
| Ukucaciswa | Ayinayo i-cross-reactivity namanye amagciwane (njenge-herpesvirus yabantu 1, 2, 3, 6, 7, 8, hepatitis B, cytomegalovirus, influenza A, njll.) noma amagciwane (Staphylococcus aureus, Candida albicans, njll.) |
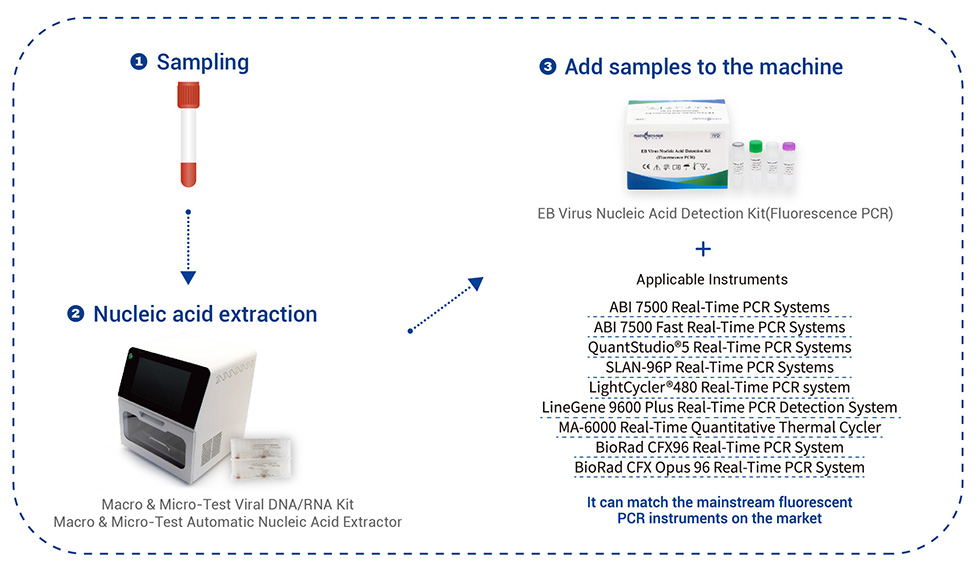
| Izinsimbi Ezisebenzayo | Ingakwazi ukufanisa amathuluzi e-PCR e-fluorescent avamile emakethe. SLAN-96P Real-Time PCR Systems I-ABI 7500 Real-Time PCR Systems I-QuantStudio®5 Real-Time PCR Systems I-LightCycler®480 Real-Time PCR Systems LineGene 9600 Plus Real-Time PCR Detection Systems I-MA-6000 Real-Time Quantitative Thermal Cycler |
Isixazululo se-PCR esiphelele
Bhala umlayezo wakho lapha futhi usithumelele wona